Enhanced TDS
Identification & Functionality
- Active Minerals
- Chemical Family
- Chemical Name
- Ingredient Name
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Food Ingredients Features
- Product Benefits
- Maintenance of normal bones and teeth
- Normal energy-yielding metabolism
- Electrolyte balance
- Normal muscle function (including the heart muscle)
- Normal functioning of the nervous system
- Role in the process of cell division
- Normal protein synthesis
- Reduction of tiredness and fatigue
- Normal psychological function
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Application Information
- Due to their neutral taste and ease of use, they are a preferred source for magnesium in food, beverages, nutritional supplements and pharmaceuticals.
- Agglomerated forms of trimagnesium citrate allow the direct compression of tablets.
- Being an excellent desiccant, it is commonly used to stabilise dry blends and to protect water sensitive ingredients.
Properties
- Physical Form
- Soluble In
- Taste
- Neutral, slightly buttery
- Soluble in
- Water, Diluted Acid
- Insoluble in
- Ethanol (96%)
- SDS Physical and Chemical Properties
Value Units Test Method / Conditions Partition Coefficient (-)1.8 to (-)0.2 - - Molecular formula (C₆H₅O₇)₂Mg₃ - - Particle Size 0.01 - 1 mm - Relative Density (at 20°C) 1.7 - 2 - - Solubility in Water (at 25°C) 200 g/L - Bulk Density 400 - 800 kg/m³ - Molecular Weight 451.12 g/mol - pH (at 5% Concentration) 5 - 9 - - Lower Explosive limits 500000 mg/m³ - Insoluble in Ethanol - - Decomposition Temperature min. 200 °C - Melting Point min. 200 °C - Odor Odorless - - Appearance Powder - - Chemical Nature Solid - - Dust Explosion Class St1 - - Color White - - - Specifications
Value Units Test Method / Conditions Sulfate Content max. 2000 mg/kg - Calcium Content max. 2000 mg/kg - Iron Content max. 100 mg/kg - Loss on Drying max. 2 % - Chloride Content max. 500 mg/kg - Arsenic Content max. 1 mg/kg - Cadmium Content max. 0.1 mg/kg - Assay (Mg, on dry substance) 15 - 16.4 % - Solubility 200 g/l - Mineral Content 16 % - - Heavy Metals
Value Units Test Method / Conditions Lead Content max. 1 mg/kg - Mercury Content max. 0.1 mg/kg CODEX STAN 193-1995, EC No. 1881/2006
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Particle Size Distribution
Particle size distribution
Particle size distribution is a very important parameter for the tableting process. Lower fine content causes less issues and cleaning effort during production which improves process times and output. Figure 1 shows the particle size distribution analysis of two tested trimagnesium citrate anhydrous granulations.
Trimagnesium citrate anhydrous GN (TMC GN) is a granulated product and has significantly lower fines and a broader particle size distribution compared to the non-granulated trimagnesium citrate anhydrous fine (TMC fine).
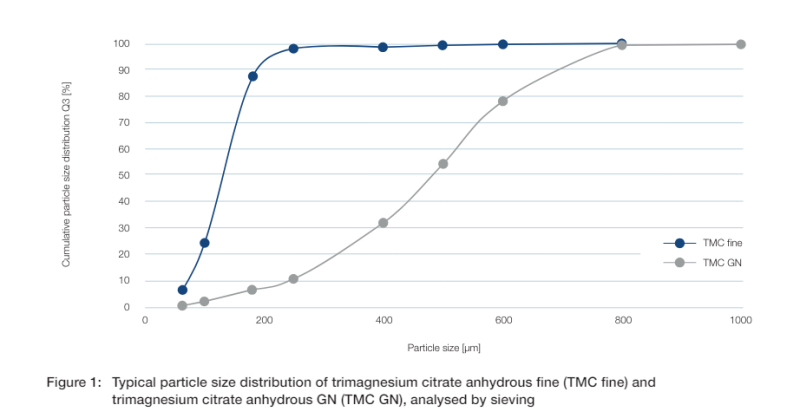
As a granulated salt, trimagnesium citrate anhydrous GN shows enhanced flowability, which improves its overall processability compared to the non-granulated product.
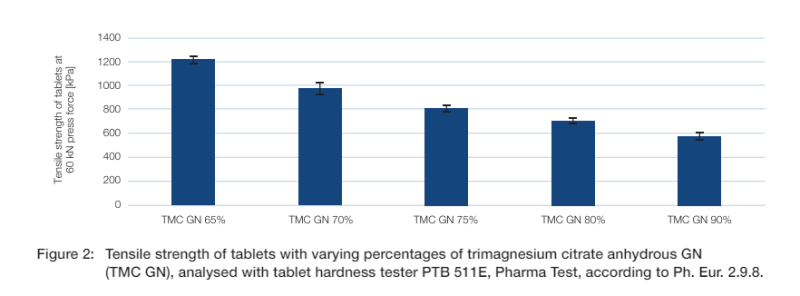
Hardness
Figure 2 shows the tensile strength of tablets with different percentages of trimagnesium citrate anhydrous GN. The tensile strength decreases as the content of trimagnesium citrate anhydrous in the tablets increases. The data shows that to achieve a tensile strength of 1.0 MPa the proportion of trimagnesium citrate anhydrous GN in the formulation must not exceed 70%. However, it is still possible to achieve almost 600 kPa with 90% trimagnesium citrate anhydrous GN using a compression force of 60 kN (236 MPa).
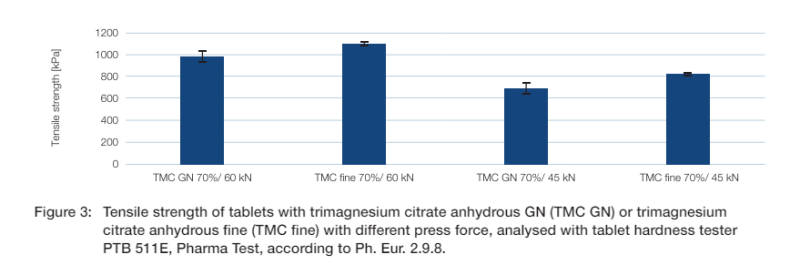
Comparing the tensile strength of tablets containing trimagnesium citrate anhydrous GN with tablets containing trimagnesium citrate anhydrous fine at 45 kN and 60 kN press force shows that there is a slight difference. The tablets with trimagnesium citrate anhydrous fine are slightly harder with the same press force (figure 3).
Packaging & Availability
- Packaging Type
- Supplied by
- Packaging Information
Trimagnesium citrate anhydrous is supplied in polyethylene lined paper bags with 20 or 25 kg net weight.
